Joseph John Thomson was born on December 18, 1856 in Cheetham, a suburb of Manchester. His father was a bookseller and publisher. It was originally intended that he should be an engineer, and, at the age of fourteen, he was sent to Owens College - later Manchester University - until there was a vacancy for an apprentice at the engineering firm selected.
After two years his father died, and his mother could not afford the large premium required for the apprenticeship. He therefore gave up engineering and in 1876 came to Trinity College, Cambridge to read Mathematics. In those days the Wranglers (undergraduates with First Class in the Mathematics Tripos) were placed in order of merit, and there was great competition to come top of the list. Thomson took the Tripos in 1880 and was placed second. The Senior Wrangler that year was Joseph Larmor, who became famous later for his contributions to theoretical physics.
The following year Thomson submitted a dissertation for the Fellowship Election at Trinity. He entered without telling his tutor, who on learning of his candidacy told him he was wasting his time - `that is just like you Thomson, never asking my advice.' He was elected.
He began work in the Cavendish Laboratory in 1880 under Lord Rayleigh, the second Cavendish Professor, and, when Rayleigh resigned the Cavendish chair in 1884, Thomson was elected to it, despite the fact that he was only 28 at the time, and was known more for his mathematical ability than for his skill in experimental physics. The appointment was not universally approved. A College tutor remarked that things had come to a pretty pass when boys were made Professors. Glazebrook, a demonstrator at the Cavendish Laboratory, wrote to Thomson `Forgive me if I have done wrong in not writing before to wish you happiness and success as Professor. The news of your election was too great a surprise to me to permit me to do so.'
Despite these misgivings, the appointment of Thomson to the Cavendish chair proved to be an inspired choice. He started experiments on the discharge of electricity through gases at low pressure, a subject which he pursued for the rest of his working life. It led to the discovery of the electron in 1897, one of the most significant events in science. The experiments leading to the discovery are described below.
Under Thomson's leadership the Cavendish Laboratory continued to make fundamental discoveries. Thomson's further work on gas discharges led to Aston's mass spectrometer, and the discovery of isotopes. Thomson received the Nobel Prize in 1906 for `his theoretical and experimental researches on the discharge of electricity through gases'. He was knighted in 1908 and received the Order of Merit in 1912. He was the President of the Royal Society from 1915 until 1920, and the Master of Trinity from 1918 until his death on August 30, 1940.
After his death Lawrence Bragg said `He, more than any other man, was responsible for the fundamental change in outlook which distinguishes the physics of this century from that of the last.'
The discovery of the electron
The electron plays a fundamental role in every branch of pure and applied science, and its discovery by Thomson marked a major advance in our understanding of nature. The following is a brief account of the background to his work and of the experiments leading to the discovery.
Under normal conditions a gas is a poor conductor of electricity. However, if the gas in a glass container is at a reduced pressure, and a voltage is applied across two electrodes inside the container, a discharge occurs and the gas becomes conducting. Streams of bright lines are observed to come from the cathode, the negative electrode; they are known as cathode rays. From the time of their discovery by the German physicist Plucker in 1858 there was much controversy over the nature of the cathode rays. Most of the German physicists thought they were some form of radiation, whereas the majority of British physicists thought they were streams of negatively charged particles. In 1897 Thomson carried out a series of experiments which demonstrated conclusively that the second view is correct.
A key observation made by Thomson was that the cathode rays are deflected by an electric field. Hertz had previously tried and failed to observe such a deflection, which gave support to the view that the cathode rays are not electric particles. Thomson realised that the reason for Hertz's failure was that the gas in his container was not at a sufficiently low pressure. Consequently, positive and negative ions in the gas neutralised the electric field that Hertz was applying. Thomson reduced the pressure and observed a deflection.
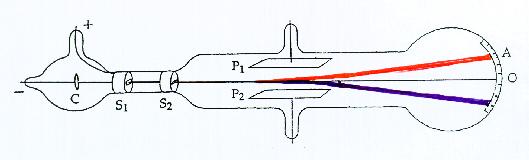
The basic features of Thomson's apparatus are shown in the diagram above. The cathode C is at a negative potential of several hundred volts, and the anode S1 is earthed. The cathode rays travel towards the anode and pass through a slit in it. They continue through a second slit in the plug S2, and travelling in a straight line (shown black in the diagram) strike the end of the tube at the point O, where they produce a narrow well-defined phosphorescent patch. P1 and P2 are a pair of parallel metal plates across which a potential difference may be applied. This gives rise to an electric field in the space between them along which the cathode rays are travelling. If the plate P1 is positive the cathode rays are deflected upwards. They follow the path shown in red and produce a phosphorescent patch at the point A. A scale is pasted on the outside of the tube to measure the amount of the deflection.
Instead of an electric field, a magnetic field may be used to deflect the particles. This is done by placing two coils (not shown in the diagram) on either side of the discharge tube in the region of P1 and P2. When a current flows through the coils a magnetic field is produced perpendicular to the previous electric field and to the direction of the cathode rays. Its direction is such that the cathode rays are deflected downwards and follow the blue path in the diagram.
Thomson determined the velocity of the cathode rays by applying the electric and magnetic fields simultaneously and adjusting their relative magnitudes so that the deflections they produced were equal and opposite. The cathode rays were then undeflected and travelled along the black line to reach the point O. The force on a charge e in an electric field E is Ee, while the force due to a magnetic field B, when the charge is moving with velocity v, is Bev. The electric and magnetic fields were made to act over the same path length d of the rays. Since the two deflections are equal, so are the two forces, i.e. Ee = Bev, and the velocity of the particles is given by the simple relation v =E/B. Thomson then measured the deflection produced by the electric field alone. This, combined with the values of E, v and d, gave the value of the ratio of the charge e to the mass m of the particles.
Thomson's claim to be the discoverer of the electron rests on two key observations. First, he found that the value of e/m was of the order of 1000 times larger than its value for the lightest particle then known, which was the hydrogen ion in electrolysis. On the assumption that the charge was the same for both particles, this meant that the mass of the new particle was of the order of 1000 times less than that of the hydrogen atom. (We now know that it is 1837 times less.) Secondly, he repeated the measurements for different gases and for different materials for the electrodes, and found that the value of e/m was independent of both the nature of the gas and the material of the electrode. In other words the particle he had discovered was a universal constituent of matter. He summarised his conclusions in the Philosophical Magazine of October 1897:
"We have in the cathode rays, matter in a new state, a state in which the subdivision of matter is carried very much farther than in the ordinary gaseous state; a state in which all matter is of one and the same kind; this matter being the substance from which all the chemical elements are made up."
Thomson made the first announcement of the existence of the electron - or `corpuscle' as he called it - at a Friday Evening Meeting of the Royal Institution on April 30, 1897. The word electron, coined originally by Johnstone Stoney in 1891 and used in another context, was applied almost immediately by other scientists to Thomson's corpuscle, but Thomson himself did not adopt the universal usage until almost twenty years later.
© Copyright 1997, G.L. Squires.
