Persons involved
Cambridge: Dr P. Cicuta, Dr C. MacPhee
KAIST: Prof. G. Yoon, Prof K-S Soh.
Existing apparatus
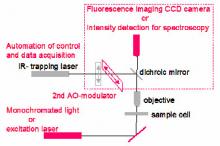
An Optical Tweezer setup (based on Elliot Scientific E-3202 system) is currently available. It is based on a single beam 1W Yb laser, which is can be positioned along a line in the focal plane through the use of an acousto-optical beam deflector (AO-modulator). The radiation pressure of the beam can trap objects having a higher refractive index than the surrounding medium. The position of trapped beads is monitored by conventional microscopy.
Proposed Experiments
We propose to explore the use of Optical Tweezers to study optical and mechanical properties of some biological systems. These experiments require upgrading the Optical Tweezer (see Figure), integrating additional optical components to enable the instrument to perform optical spectroscopy and micro-mechanical measurements.
Optical imaging and spectroscopy
The optical trap allows to position and hold a micro biological object such as cells and other structures. This enables the visualization of processes evolving in time and the imaging of dynamical response following some perturbation. In the initial stages of this research we want to prove the possibility of manipulating different biological structures, with the aim of performing micro-spectroscopy on such systems. This is of great interest because it opens the possibility of single-cell measurements, as opposed to a volume average result as in conventional spectroscopy. For example it would be possible to measure the difference in oxygen uptake between single red blood cells.
Mechanical Measurements
A)We are interested in the mechanical properties of biological systems, such as cell membranes and protein filaments (like microtubules and amyloid fibrils). These can be accessed through the monitoring of thermal fluctuations of trapped objects. In this case we require visualisation of the membrane or filament as well as the trapped bead. This can be done straightforwardly in fluorescence microscopy, by integrating an excitation beam into the existing optical setup. A camera with particularly high sensitivity will be required to image single filaments.
B) With the addition of a second AO modulator the trapped bead can be positioned not just along a line but over the whole x-y plane. This makes it possible to explore the swimming motion of a semi-flexible filament, where one end is made to oscillate perpendicular to the filament direction, resulting in motion along the filament axis. This problem, involving both fluid dynamics and the mechanical behaviour of a thin filament, is of biological relevance as well as of fundamental physical interest.